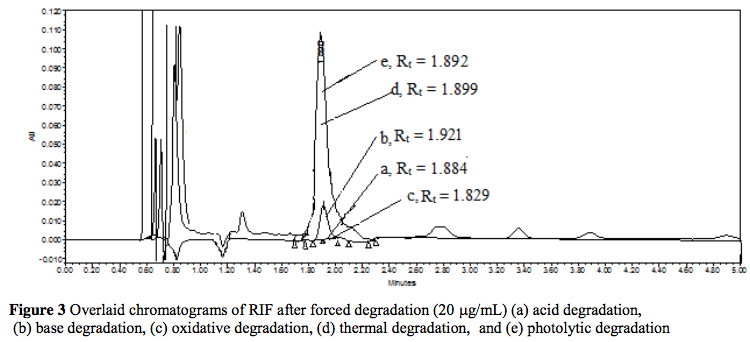
Development and validation of a stability-indicating ultra-performance liquid chromatographic method for the determination of rifampicin in bulk drug and capsules
Abstract
Method: A discrete choice experiment (DCE) was conducted in general population. The five relevant MTM service attributes (service setting, service provider, length of service, frequency of follow up and service fee) were identified from literature reviews, face-to-face interview and survey. The DCE included 7 choice tasks composed of five attributes, two service profiles, and none option using a statistically efficient design. Six questionnaire sets were randomly assigned to 346 samples. The multinomial logistic regression was used to estimate preferences and WTP.
Results: The totals of 265 questionnaires were included in the analysis. All five attributes had statistically significant impact on respondents’ utility of MTM service (p<0.05). MTM service at drugstore was preferred to home visit and services provided by the same pharmacist was preferred to any available pharmacist. Moreover shorter length of service and follow up with less frequency were preferred. The highest utility model was MTM service provided by the same pharmacist at the drugstore with 20 minutes length of service, 10 weeks follow up, and 150 baht service fee.
Conclusions: MTM service was beneficial and valued by consumers. The WTP and attributes obtained from the study could be used to design pharmacy service benefit package to match with consumer needs and characteristics as well as the amount of reimbursement for pharmacy services.
Full Text:
41-48:PDFReferences
A. G. Gilmann, L. S. Goodmann. In the Pharmaceutical Basis of Therapeutics, 4th Edn. The Mc Millan Co. 1333, 1970.
J. Melnick. Adelbergs, Med. Microbio. 21: 171- 1998.
P. F. Barnes and S. A. Barrows. Tuberculosis in 1990s, Ann. Intern. Med. 119: 400-410, 1993.
S. Oldfield, J. D. Borg, H. J. Stiles and B. M. Buckley. Measurement of rifampicin and 25-desacetylrifampicin in biological fluids using high- performance liquid chromatography with direct sample injection, J. Chrom. 377: 423-429, 1986.
A. B. M. Jamaluddin, G. Sarwar, M. A. Rahim and M. K. Rahman. High-performance liquid chromatographic assay of rifampicin in human serum, J. Chrom. 525: 495-497, 1990.
G. C. He ́ctor and O. C. Alejandro. Simultaneous determination of rifampicin, isoniazid and pyrazinamide in tablet preparations by multivariate spectrophotometric calibration, J. Pharm. Biomed. Anal. 20: 681–686, 1999.
M. A. Espinosa, V. M. I. Acedo, D. P. A. Muñoz, F. Salinas and C. F. Cañada. Comparative study of partial least squares and a modification of hybrid linear analysis calibration in the simultaneous spectrophotometric determination of rifampicin, pyrazinamide and isoniazid, Anal. Chim. Acta. 427: 129–136, 2001.
S. A. Benetton, E. R. M. Kedor-Hackmann, M. I. R. M. Santoro and V. M. Borges. Visible spectrophotometric and first-derivative UV spectrophotometric determination of rifampicin and isoniazid in pharmaceutical preparations, Talanta. 47: 639–643, 1998.
A. R. Rote and A. K. Sharma. Simultaneous spectrophotometric determination of rifampicin, isoniazid and pyrazinamide by first-derivative UV spectrophotometry in combined pharmaceutical dosage forms, Indian J. Pharm. Sci. 59: 119-123, 1997.
M. Y. Rasha and M. M. Hadir. A new hybrid double divisor ratio spectra method for the analysis of ternary mixtures, Spectrochim. Acta. Part A. 70: 1152–1166, 2008.
L. F. Marcellos, A. F. Faria, M. V. N. Souza, M. R. Almeida, G. P. Sabin, R. J. Poppi and M. A. L. Oliveira. Simultaneous analysis of first- line anti-tuberculosis drugs in tablets by UV spectrophotometry compared to capillary zone electrophoresis, Cent. Eur. J. Chem. 10: 1808-1816, 2012.
R. B. Kakde, A. V. Kasture and S. G. Wadodkar. Spectrophotometric determination of rifampicin and isoniazid in pharmaceutical preparations, Indian J. Pharm. Sci. 64: 24-27, 2002. [13]T. Wahdan. Voltammetric method for the simultaneous determination of rifampicin and isoniazid in pharmaceutical formulations, Chem. Anal. (Warsaw). 50: 457-464, 2005.
A. Z. Karim and S. A. Payam. Simultaneous polarographic determination of isoniazid and rifampicin by differential pulse polarography method and support vector regression, Electrochim. Acta. 55: 6570–6576, 2010.
M. A. L. Alonso, O. R. Dom ́ınguez and M. J. M. Arcos. Resolution of ternary mixtures of rifampicin, isoniazid and pyrazinamide by differential pulse polarography and partial least squares method, Anal. Chim. Acta. 449: 167–177, 2001.
M. A. A. Alonso, J. M. Kauffmann and M. J. M. Arcos. HRP-based biosensor for monitoring rifampicin, Biosen. Bioel. 18: 1165-1171, 2003. [17] L. Baoxin, H. Yuezhen, L. Jiagen and Z. Zhujun. Simultaneous determination of rifampicin and isoniazid by continuous-flow chemiluminescence with artificial neural network calibration, Anal. Bioanal. Chem. 383: 817–824, 2005.
S. A. Halvatzis, P. M. M. Timotheou and T. P. Hadjiioannou. Continuous-flow chemiluminometric determination of dihydralazine, rifampicin and rifamycin SV by oxidation with N-bromosuccinimide, Anal. Chim. Acta. 272: 251-263, 1993.
D. H. Shewiyoa, E. Kaaleb, P. G. Rishab, B. Dejaegherc, V. J. Smeyers and H. Y. Vander. Optimization of a reversed-phase-high- performance thin-layer chromatography method for the separation of isoniazid, ethambutol, rifampicin and pyrazinamide in fixed-dose combination antituberculosis tablets, J. Chromatogr. A. 1260: 232–238, 2012.
J. Ali, N. Ali, Y. Sultana, S. Baboota and S. Faiyaz. Development and validation of a stability-indicating HPTLC method for analysis of antitubercular drugs, Acta. Chromatogr. 18: 168-179, 2007
S. Gunasekaran and E. Sailatha. Estimation of pyrazinamide, isoniazid and rifampicin in pharmaceutical formulations by high performance liquid chromatography method, Asian J. Chem. 21: 3561- 3566, 2009.
E. Calleri, E. De Lorenzi S. Furlanetto G. Massolini and G. Caccialanza. Validation of a RP-LC method for the simultaneous determination of isoniazid, pyrazinamide and rifampicin in a pharmaceutical formulation, J. Pharm. Biomed. Anal. 29: 1089–1096, 2002.
S. Sadeghi and E. Karimi. Spectrophotometric determination of rifampicin through chelate formation and charge transfer complexation in pharmaceutical preparation and biological fluids, Chem. Pharm. Bull. 54: 1107-1112, 2006.
C. S. P. Sastry, T. E. Divakar and U. V. Prasad. Spectrophotometric determination of rifampicin with uranyl or thorium(IV), J. Inst. Chem. (India) 58: 17-18, 1986.
C. S. P. Sastry, T. E. Divakar and U. V. Prasad. Spectrophotometric determination of rifampicin with some metal ions, Indian Drugs. 22: 604-606, 1985.
T. P. Gandhi, A. A. Patel, P. R. Patel and V. C. Patel. Colorimetric estimation of rifampicins in formulations and biological fluids by metallic ions, Indian Drugs 16: 10-12, 1978.
B. S. Reddy and C. S. P. Sastry. Ion-pair extraction method for ethambutol, ethionamide and rifampicin determination, J. Inst. Chem. (India) 55: 69-70, 1983.
M. G. Shereen, M. B. Salah and M. E. Abdel-Hamid. Comparative spectrophotometrc analysis of rifampicin by chelate formation and charge-transfer complexation, Anal. Lett. 25: 725-743, 1992.
I. C. Shukla, P. K. Dwivedi, S. Kumar, B. K. Singh and A. Dubey. Application of ammonium metavanadate for the determination of some tubercular and adrenocortical steroid drugs, J. Ind. Chem. Soc. 84: 100- 102, 2007.
C. S. P. Sastry, T. E. Divakar and U. V. Prasad. Spectrophotometric determination of rifampicin with chloranil, Indian J. Pharm. Sci. 47: 45- 46, 1985.
T. E. Divakar, U. V. Prasad and C. S. P. Sastry. Spectrophotometric estimation of tetracyclines and rifampicin using p-N,N- dimethylphenylenediamine and chloramine T, Indian Drugs 22: 328- 329, 1985.
G. R. Rao, S. S. N. Murty and E. V. Rao. Spectrophotometric determination of rifampicin in pharmaceutical dosage forms, Indian Drugs 22: 484-488, 1985.
T. E. Divakar, S. Sunitha, G. K. Deepthi, T. Benzamin and N. P. Babu. Assay of rifampicin in bulk and its dosage forms by visible spectrophotometry using chloranilic acid, Int. J. Chem. Environ. Pharm. Res. 3: 64-67, 2012.
N. B. Barsoum, M. S. Kamel and M. M. A. Diab. Spectrophotometric determination of isoniazid and rifampicin from pharmaceutical preparations and biological fluids, Res. J. Agri. Bio. Sci. 4: 471-484, 2008.
A. A. Salem, H. A. Mossab and B. N. Barsoum. Quantitative determinations of levofloxacin and rifampicin in pharmaceutical and urine samples using nuclear magnetic resonance spectroscopy, Spectrochim. Acta Part A. 62: 466–472, 2005.
H. Younghee and S. Sunmi. Electrochemical behaviour and differential pulse polarographic determination of rifampicin in the pharmaceutical preparations, Arch. Pharm. Res. 24: 100-104, 2001. [37]M. A. A. Lomillo, O. D. Renedo and M. J. A. Martinez. Optimization procedure, applying the experimental-design methodology, for the determination of rifampicin after metal complexation by differential pulse adsorptive stripping voltammetry, Helvet. Chim. Acta. 85: 2430-2439, 2002.
Y. Shah, S. Khanna, K. C. Jindal and V. S. Dighe. Determination of rifampicin and isoniazid in pharmaceutical formulations by HPLC, Drug. Dev. Ind. Pharm. 18: 1589-1596, 1992.
L. Novakova, L. Matysova, and P. Solich. Advantages of applications of UPLC in pharmaceutical analysis, Talanta. 68: 908-918, 2006.
ICH [Stability Testing of New Drug Substances and Products (Q1AR2), International Conference on Harmonization, Food and Drug Administration, USA, November 1996 and February 2013.
ICH [Validation of Analytical Procedures: Methodology (Q2AR1), International Conference on Harmonization, Food and Drug Administration, USA, November 1991 and November 2005.
M. Bakshi and S. Singh. Development of validated stability- indicating assay methods-critical review, J. Pharm. Biomed. Anal. 28: 1011-1040, 2006.
ICH Harmonized Tripartite Guidelines, Validation, Analytical Procedures, Text and Methodology, Q2 (R1), Geneva, Switzerland, 2005.
Refbacks
- There are currently no refbacks.

