In vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease
Abstract
Black pepper oil and its major components were investigated for the biological activities related to the prevention of AD. The inhibition of acetylcholinesterase (AChE) and β-amyloid aggregation, anti-inflammatory and antioxidant activities were examined. GC-MS analysis of black pepper oil identified 33 constituents with δ-3- carene, limonene, (-)-β-pinene, α-pinene and caryophyllene as the main components. The AChE inhibitory activity by microplate assay revealed that black pepper oil exhibited potent AChE inhibition with an IC50 value of 5.97 μg/ml. Among the compounds tested, δ-3-carene exhibited AChE inhibitory activity with an IC50 value of 20.50 μg/ml. β-Amyloid aggregation inhibitory activity was determined by a thioflavin T assay. Black pepper oil exhibited a weak inhibitory activity with 33.17 ± 6.67 % inhibition at 100 μg/ml, while limonene possessed stronger inhibitory activity with an IC50 value of 3.77 μg/ml. Anti-inflammatory activity was determined by a COX-2 activity assay in THP-1 cells. The oil and caryophyllene showed strong COX-2 inhibition. The weak antioxidant activity of black pepper oil and its major components was observed in TLC/DPPH, microplate assays and ESR analysis. These findings suggest that black pepper oil may be beneficial in lowering the risk of Alzheimer’s disease via AChE inhibition and anti-inflammatory activity via COX-2 inhibition.
Full Text:
94-101:PDFReferences
T. S. Anekonda, and P. H. Reddy. Can herbs provide a new generation of drugs for treating Alzheimer’s disease?, Brain Res. Rev. 50: 361-576 (2005).
M. D. Carter, G. A. Simms, and D. F. Weaver. The development of new therapeutics for Alzheimer’s disease, Clin. Pharmacol. Ther. 88: 475-486 (2010).
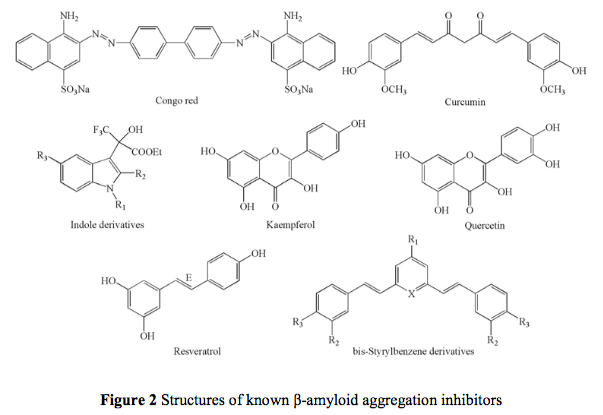
M. Catto, R. Aliano, A. Carotti, S. Cellamare, F. Palluotto, R. Purgatorio, A. De Stradis, and F. Campagna. Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1,3-diones and indol-2-aryldiazenylmethylene-3-ones as β-amyloid aggregation inhibitors, Eur. J. Med. Chem. 45: 1359-1366 (2010).
M. A. Smith, C. A. Rottkamp, A. Nunomura, A. K. Raina, and G. Perry. Oxidative stress in Alzheimer’s disease, Biochim. Biophys. Acta 1502: 139-144 (2000).
E. G. McGeer, and P. L. McGeer. Inflammatory process in Alzheimer’s disease, Prog. Neuro-Psychoph. 27: 741-749 (2003).
J. Vina, A. Lloret, R. Orti, and D. Alonso. Molecular bases of the treatment of Alzheimer’s disease with antioxidants: prevention of oxidative stress, Mol. Aspects Med. 25: 117-123 (2004).
M. M. Essa, R. K. Vijayan, G. Castellano-Gonzalez, M. A. Memon, N. Braidy, and G. J. Guillemin. Neuroprotective effect of natural products against Alzheimer’s disease, Neurochem. Res. 37: 1829- 1842 (2012).
P. J. Houghton, Y. Ren, and M. J. Howes. Acetylcholinesterase inhibitors from plants and fungi, Nat. Prod. Rep. 23: 181-199 (2006).
W. Kitphati, K. Wattanakamolkul, P. Lomarat, P. Phanthong, N. Anantachoke, V . Nukoolkarn, K. Thirapanmethee, and N. Bunyapraphatsara. Anticholinesterase of essential oils and their constituents from Thai medicinal plants on purified and cellular enzymes, JAASP 1: 58-67 (2012).
M. Miyazawa, H. Watanabe, and H. Kameoka. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton, J. Agric. Food Chem. 45: 677-679 (1997).
S. Dohi, M. Terasaki, and M. Makino. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oil, J. Agric. Food Chem. 57: 4313-4318 (2009).
M. Miyazawa, and C. Yamafuji. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids, J. Agric. Food Chem. 53: 1765- 1768 (2005).
W. J. Yoon, N. H. Lee, and C. G. Hyun. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2,and pro-inflammatory cytokines in RAW 264.7 macrophages, J. Oleo Sci. 59: 415-421 (2010).
J. Y. Zhou, F. D. Tang, G. G. Mao, and R. L. Bian. Effect of α- pinene on nuclear translocation of NF-κB in THP-1 cells, Acta Pharmacol. Sin. 25: 480-484 (2004).
E. S. Fernandes, G. F. Passos, R. Medeiros, F. M. da Cunha, J. Ferreira, M. M. Campos, L. F. Pianowski, and J. B. Calixto. Anti- inflammatory effects of compounds alpha-humulene and (−)-trans- caryophyllene isolated from the essential oil of Cordia verbenacea, Eur. J. Pharmacol. 569: 228-236 (2007).
S. Aazza, B. Lyoussi, and M. G. Miguel. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds, Molecules 16: 7672-7690 (2011).
M. Lahlou. Essential oils and fragrance compounds: bioactivity and mechanisms of action, Flavour Frag. J. 19: 159-165 (2004).
I. K. Rhee, M. van de Meent, K. Ingkaninan, and R. Verpoorte. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining, J. Chromatogr. A 915: 217-223 (2001).
G. L. Ellman, K. D. Courtney, V. Andres, and R. M. Featherstone. A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol. 7: 88-90 (1961).
K. Ingkaninan, P. Temkitthawon, K. Chuenchom, T. Yuyaem, and W. Thongnoi. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies, J. Ethnopharmacol. 89: 261-264 (2003).
M. Bourhim, M. Kruzel, T. Srikrishnan, and T. Nicotera. Linear quantitation of Aβ aggregation using Thioflavin T: Reduction in fibril formation by colostrinin, J. Neurosci. Meth. 160: 264-268 (2007).
A. A. Reinke and J. E. Gestwicki. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility, Chem. Biol. Drug. Des. 70: 206-215 (2007). [23] F. Zhao, L. Wang, and K. Liu. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway, J. Ethnopharmacol. 122: 457-462 (2009).
M. A. Saleh, S. Clark, B. Woodard, and S. A. Deolu-Sobogun. Antioxidant and free radical scavenging activities of essential oils, Ethnic. Dis. 20: 78-82 (2010).
F. Nanjo, K. Goto, R. Seto, M. Suzuki, M. Sakai, and Y. Hara. Scavenging effects of tea catechins and their derivatives on 1,1- diphenyl-2-picrylhydrazyl radical, Free Radical Bio. Med. 21: 895-902 (1996).
H. Schulz, M. Baranska, R. Quilitzsch, W. Schütze, and G. Lösing. Characterization of peppercorn, pepper oil, and pepper oleoresin by vibrational spectroscopy methods, J. Agric. Food Chem. 53: 3358- 3363 (2005).
S. D. Giovanni, A. Borloz, A. Urbain, A. Marston, K. Hostettmann, P. A. Carrupt, and M. Reist. In vitro screening assays to identify natural or synthetic acetylcholinesterase inhibitors: thin layer chromatography versus microplate methods, Eur. J. Pharm. Sci. 33: 109- 119 (2008).
Y. Porat, A. Abramowitz, and E. Gazit. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism, Chem. Biol. Drug Des. 67: 27-37 (2006).
P. Campiglia, C. Esposito, M. Scrima, I. Gomez-Monterrey, A. Bertamino, P. Grieco, E. Novellino, and A. M. D′Ursi. Conformational stability of Aβ-(25-35) in the presence of thiazolidine derivatives, Chem. Biol. Drug Des. 69: 111-118 (2007).
M. A. Ocete, S. Risco, A. Zarzuelo, and J. Jimenez. Pharmacological activity of the essential oil of Bupleurum gibraltaricum: anti-inflammatory activity and effects on isolated rat uteri, J. Ethnopharmacol. 25: 305-313 (1989).
I. Orhan, E. Küpeli, M. Aslan, M. Kartal, and E. Yesilada. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L., J. Ethnopharmacol. 105: 235- 240 (2006).
I. Lorente, M. A. Ocete, A. Zarzuelo, M. M. Cabo, and J. Jimenez. Bioactivity of the essential oil of Bupleurum fruticosum, J. Nat. Prod. 52: 267-272 (1989).
S. Bourgou, A. Pichette, B. Marzouk, and J. Legault. Bioactivities of black cumin essential oil and its main terpenes from Tunisia, S. Afr. J. Bot. 76: 210-216 (2010).
X. J. Duan, W. W. Zhang, X. M. Li, and B. G. Wang. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata, Food Chem. 95: 37-43 (2006).
L. L. Mensor, F. S. Menezes, G. G. Leitão, A. S. Reis, T. C. dos Santos, C. S. Coube, and S. G. Leitão. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method, Phytother. Res. 15: 127-130 (2001).
I. P. S. Kapoor, B. Singh, G. Singh, C. S. deHeluani, M. P. deLampasona, and C. A. N. Catalan. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum), J. Agric. Food Chem. 57: 5358-5364 (2009).
W. Brand-Williams, M. E. Cuvelier, and C. Berset. Use of a free radical method to evaluate antioxidant activity, Lebensm. Wiss. Technol. 28: 25-30 (1995).
Refbacks
- There are currently no refbacks.

