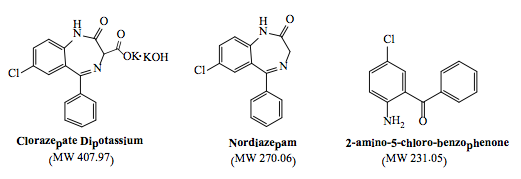
Development and Validation of a Stability-Indicating HPLC Method for Determination of Clorazepate Dipotassium and Its Main Impurities in Bulk Drug and Capsules
Abstract
A simple isocratic stability-indicating HPLC method was developed and validated for the simultaneous
determination of clorazepate dipotassium in the presence of its main impurities; nordiazepam and 2-amino-5-
chlorobenzophenone, in bulk drug and capsules. The chromatographic analysis was performed on a Zorbax
Eclipse XDB-C18 column (75 mm x 4.6 mm i.d., 3.5 mm) using a mobile phase consisting of 5 mM ammonium
formate in methanol and 5 mM ammonium formate in water (65: 35, v/v) at a flow rate of 0.7 mL/min and UV
detection at 230 nm. The forced degradation studies were performed under various conditions according to the
ICH guidelines. The degradation products from the studies were investigated by HPLC and, later, by tandem
LC-MS. The validation tests including specificity, linearity, accuracy, precision, LOD and LOQ were
performed. The calibration curves of the drug and the two related substances were linear in the concentration of
2 to 100 g/mL (r2 = 0.9990), 2-50 g/mL (r2 = 0.9995) and 0.4-25 g/mL (r2 = 0.9993), respectively. The
proposed method was proven to be suitable for the quantitative determination and stability studies of clorazepate
dipotassium in bulk drug and capsules.
Full Text:
127-140:PDFRefbacks
- There are currently no refbacks.

