HPTLC method for simultaneous estimation of ramipril and losartan potassium in pharmaceutical dosage form: development and validation consideration
Abstract
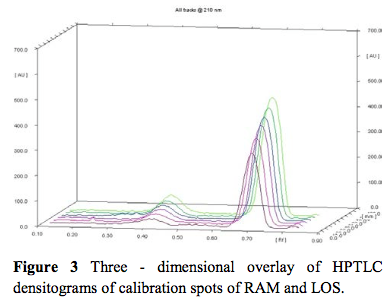
This paper describes validated high-performance thin-layer chromatography (HPTLC) methods for simultaneous estimation of ramipril (RAM) and losartan potassium (LOS) in pure powder and formulation. The HPTLC separation was achieved on an aluminum-backed layer of silica gel 60F254 using methanol: ethyl acetate: toluene: glacial acetic acid (1:9:1:0.2 v/v/v/v) as mobile phase. Quantification in HPTLC method was achieved with UV detection at 210 nm over the concentration range of 300 – 1300 ng/spot for RAM and 3000 – 13000 for LOS, respectively, with recovery of 98.93 - 99.73 and 98.96 - 100.11 % for RAM and LOS, respectively. These methods are simple, specific, precise, sensitive and robust; they are applicable for the simultaneous determination of RAM and LOS in pure powder and formulation.
Full Text:
83-88:PDFReferences
S. A. Kumar, M. Debnath, and J.V.L.N.S. Rao. Simultaneous estimation of losartan potassium, ramipril and hydrochlorothiazide in bulk as well as in pharmaceutical formulation by RP-HPLC. Indo Am. J. Pharm. Res. 3: 3871–3894 (2013).
M. M. Baig, V. V. Vaidya, R. T. Sane, S. N. Menon, and K. Dalvi. Simultaneous RP-LC for losartan potassium, ramipril and hydrochloro- thiazide in pharmaceutical preparation. Chromatographia 642: 293–296 (2006).
M. Gandhimathi. HPLC determination of losartan potassium and ramipril in tablets. Ind. Drugs. 41: 120–122 (2004).
K. S. Rao, and K. Srinivas. RP-HPLC method for the determination of losartan potassium and ramipril in combined dosage form Indian J. Pharm. Sci. 72: 108–111 (2010).
L. H. Deanne and F. Jennifer. Development and validation of stability indicating HPLC method for the simultaneous determination of losartan potassium, hydrochlorthizide and their degradation products. J. Pharm. Boimed. Anal. 42: 411–422 (2006).
B. N. Suhagia, R. R. Shah, and D. M. Patel. Development of RP- HPLC method for evaluating losartan potassium and hydrochloro-thizide tablets. Ind. J. Pharm. Sci. 67: 37–42 (2005).
A. I. Patel, C. K. Oza, J. P. Prajapati, A. J. Vyas, and P. Mehta. RP-HPLC method for the determination of losartan potassium and perindopril erbumine in combined tablet dosage form. Int. J. Pharm. Biosci. 2: 709–715 (2011).
N. Erk. Analysis of binary mixtures of losartan potassium and hydrochlorthizide by using high performance liquid chromatography, ratio derivative spectroscopy and compensation technique. J. Pharm. Biomed. Anal. 24: 603–611 (2001).
A. S. Ozkan. Simultaneous determination of losartan potassium and hydrochlorthizide from tablet and human serum by RP-HPLC. J. Liq. Chromatogr. Rel. Tech. 24: 2337–2346 (2001).
P. K. Yeung, A. Jamieson, G. J. Smith, D. Fice, and P. T. Pollak. Determination of plasma concentrations of losartan in patients by HPLC using solid phase extraction and UV detection. Int. J. Pharm. 204: 17–22 (2000).
M. Ansari, M. Kazemipour, F. Khosravi, and M. Baradaran. A comparative study of first-derivative spectrophotometry and high- performance liquid chromatography applied to the determination of losartan potassium in tablets. Chem. Pharm. Bull. 52: 1166–1170 (2004). [12] N. Rahman, Y. Ahmad, and S. N. H. Azmi. Kinetic spectrophoto- metric method for the determination of ramipril in pharmaceutical formulations. AAPS Pharm. Sci. Tech. 6: E543–E551 (2005).
B. L. Hogan, M. Williams, A. Idiculla, T. Veysoglu, and E. Parente. Development and validation of a liquid chromatographic method for the determination of the related substances of ramipril in Altace capsules. J. Pharm. Biomed. Anal. 23: 637–651 (2000).
F. Belal, I. A. Al-Zaagi, E. A. Gadkariem, and M. A. Abounassif. A stability-indicating LC method for the simultaneous determination of ramipril and hydrochlorothiazide in dosage forms. J. Pharm. Biomed. Anal. 24: 335–342 (2001).
P. R. Patil, S. U. Rakesh, P. N. Dhabale, and K. B. Burade. Simultaneous estimation of ramipril and amlodipine by UV spectro- photometric method. Res. J. Pharm. Tech. 2: 304–307 (2009).
L. V. Potale, M. C. Damle, A. S. Khodke, and K. G. Bothara. A validated stability indicating HPTLC method for simultaneous estimation of ramipril and telmisartan. Int. J. Pharm. Sci. Rev. Res. 2: 35–39 (2010).
Z. Zhu, A. Vachareau, and L. Neirinck. Liquid chromatography– mass spectrometry method for determination of ramipril and its active metabolite ramiprilat in human plasma. J. Chromatogr. B. 779: 297–306 (2002).
ICH Expert Working Group. Validation of analytical procedures: text and methodology Q2(R1). Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, April 25–27, 2005.
R. B. Patel, M. R. Patel, N. Dubey, N. N. Dubey, and B. G. Patel. HPTLC method development and validation: strategy to minimize methodological failures. J. Food Drug Anal. 20: 561–571 (2012).
Parmar et al, 2015

Refbacks
- There are currently no refbacks.

